The world’s first leadless pacemaker, developed by an Indian-origin scientist Vivek Reddy, has shown promising results after one year of human trials on 32 patients who received the pacemaker.
The world’s first leadless pacemaker, developed by an Indian-origin scientist Vivek Reddy, has shown promising results after one year of human trials on 32 patients who received the pacemaker.
“This is the first time we have seen one-year follow-up data for this innovative, wireless cardiac pacing technology. Our results show the leadless pacemaker is comparable to traditional pacemakers,” said Reddy, director of arrhythmia services at the Mount Sinai Hospital here.
The findings further support the promising performance and safety of this minimally-invasive, non-surgical pacing device.
The follow-up study evaluated 32 patients with a slowed heartbeat (bradycardia) who successfully received St. Jude Medical’s “Nanostim” leadless pacemaker at two hospitals in Prague and one in Amsterdam.
“There was no experience of infections or failure to sense, pace or communicate with the pacemaker,” Reddy noted.
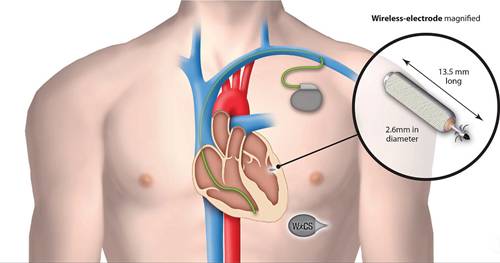
The leadless cardiac pacemaker is placed directly inside a patient’s heart without surgery during a catheter-guided procedure through the groin via the femoral vein.
The device, resembling a tiny, silver tube and smaller than a triple-A battery, is only a few centimetres in length, making it less than 10 percent the size of a traditional pacemaker.
It works by closely monitoring the heart’s electrical rhythms and if the heart beat is too slow it provides electrical stimulation therapy to regulate it.
“More long-term follow-up of these ‘leadless’ study patients will further our understanding of the potential advantages, benefits, and complication risks of leadless pacemaker technology, along with additional ongoing, larger trials,” Reddy said.
More than four million patients globally have a pacemaker, and 700,000 new patients receive one each year.
Reddy presented the one-year ‘leadless’ study data findings at ‘Heart Rhythm 2014,’ the Heart Rhythm Society’s 35th annual scientific sessions in San Francisco city in the US May 9.
Source: Times of India