Guidelines recommending annual low-dose CT lung cancer screening for older smokers have been approved by the US Preventive Services Task Force. The recommendations apply to individuals aged between 55 and 80 who are at high risk for lung cancer as a result of heavy smoking.
The guidelines are published in the journal Annals of Internal Medicine.
According to the American Cancer Society, approximately 228,190 new cases of lung cancer will have been diagnosed during 2013, with 159,480 deaths from the disease. This accounts for around 27% of all cancer deaths.
Background information from the guidelines states that around 85% of all cases of lung cancer are caused by smoking, and the risk of lung cancer increases with age, particularly for those aged over 55.
Dr. Michael LeFevre, co-vice chair of the US Preventive Services Task Force (USPSTF), says these factors suggest that the longer a person smokes, the higher their risk is for developing lung cancer.
Guidelines recommending annual low-dose CT lung cancer screening for older smokers have been approved by the US Preventive Services Task Force. The recommendations apply to individuals aged between 55 and 80 who are at high risk for lung cancer as a result of heavy smoking.
The guidelines are published in the journal Annals of Internal Medicine.
According to the American Cancer Society, approximately 228,190 new cases of lung cancer will have been diagnosed during 2013, with 159,480 deaths from the disease. This accounts for around 27% of all cancer deaths.
Background information from the guidelines states that around 85% of all cases of lung cancer are caused by smoking, and the risk of lung cancer increases with age, particularly for those aged over 55.
Dr. Michael LeFevre, co-vice chair of the US Preventive Services Task Force (USPSTF), says these factors suggest that the longer a person smokes, the higher their risk is for developing lung cancer.
He adds:
“When clinicians are determining who would most benefit from screening, they need to look at a person’s age, overall health, how much the person has smoked, and whether the person is still smoking or how many years it has been since the person quit.”
Low-dose CT scanning ‘more accurate’
The 2004 lung cancer screening recommendation from the USPSTF stated that the “evidence was insufficient to recommend for or against screening for lung cancer in asymptomatic persons with LDCT (low-dose computed tomography), chest radiography, sputum cytologic evaluation or a combination of these tests.”
With the aim of updating these recommendations, a panel from the USPSTF reviewed more than 33 studies involving current or former smokers who were at average or high risk for developing lung cancer.
The analysis included a study of more than 50,000 people who were a part of the National Lung Screening Trial.
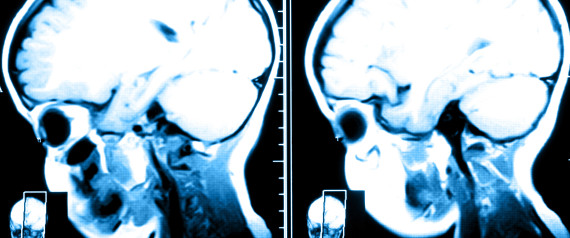
From their research, the panel found that low-dose computed tomography (CT) lung cancer screening was more accurate in identifying the disease in its early stages, compared with alternative screening tests.
Their findings have led the USPSTF to “recommend annual screening for lung cancer with low-dose computed tomography in adults aged 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years.”
A 30-pack year is the equivalent to one pack a day for 30 years, or two packs a day for 15 years.
Screening not recommended when smoking ceased for 15 years
However, they note that screening should be stopped once a person has not smoked for 15 years or develops a health problem that shortens life expectancy or the willingness or ability to undergo potential lung surgery.
Dr. Virginia Moyer, chair of USPSTF emphasizes that it is important to assess a patient’s overall health to determine whether screening is appropriate.
“The benefit of screening may be significantly less in people with serious medical problems and there is no benefit in screening someone for whom treatment is not an option,” she says.
“In these people, screening may lead to unintended harms such as unnecessary tests and invasive procedures.”
She also adds that although screening for lung cancer is beneficial, it should not be seen as an alternative to giving up smoking.
Source: medical news today


 Researchers have revealed an atomic-level view of a genetic defect that causes a form of muscular dystrophy, myotonic dystrophy type 2.
Researchers have revealed an atomic-level view of a genetic defect that causes a form of muscular dystrophy, myotonic dystrophy type 2.